
Many VOCs are carcinogens depending on exposure period and concentration that can cause cancers such as lymphatic and hematopoietic types. Due to their low boiling point and high volatility, they can be easily released into the environment. Introduction Volatile organic compounds (VOCs) are the most important groups of air pollutants and are health hazards which contain aromatics, aldehydes, ketones, alkanes, alcohols etc. The sensor shows short response/recovery time (less than two minutes), complete reversibility and repeatability which are attributed to the physisorption of the gases into the MOF pores and high stability of the device. The best sensor response is observed for pyridine detection with sensitivity of 2.793 Hz ppm −1.
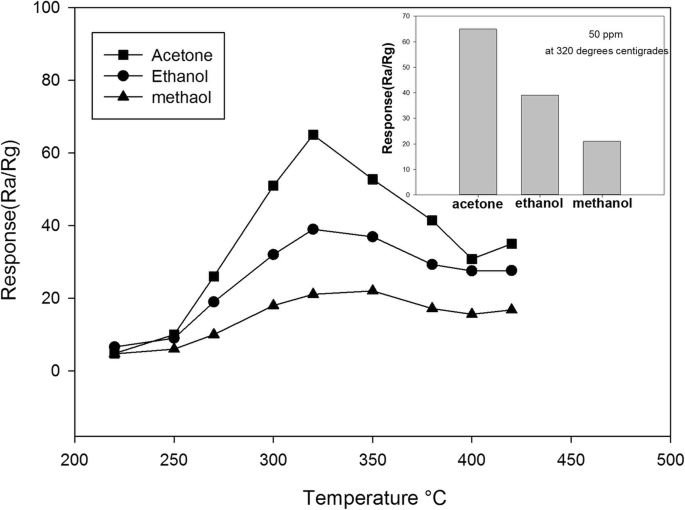
Sensing properties such as sensitivity, reversibility, stability, response time, recovery time, and limit of detection (LOD) of the sensor are investigated. Here, the sensor response to some VOCs with different functional groups and polarities, such as methanol, ethanol, isopropanol, n-hexane, acetone, dichloromethane, chloroform, tetrahydrofuran (THF), and pyridine under N 2 atmosphere at ambient conditions is studied. The frequency of the QCM crystal is changed during exposure to different concentrations of target gas molecules. A stable and uniform layer of MOF is coated onto the surface of a QCM sensor by the drop casting method. The structural and chemical properties of synthesized MIL-101(Cr) are investigated by X-ray diffraction (XRD), Fourier-transfer infrared spectroscopy (FTIR) and scanning electron microscopy (SEM) and so on. MIL-101(Cr) is selected as a good candidate from the MOFs family to fabricate a quartz crystal microbalance (QCM) nanosensor for the detection of volatile organic compound (VOC) vapors. I used to travel to Brasil a lot on business and be more fluent in Portuguese, but I haven't been there since 1993.The application of metal–organic frameworks (MOFs) as a sensing layer has been attracting great interest over the last decade, due to their high porosity and tunability, which provides a large surface area and active sites for trapping or binding target molecules.
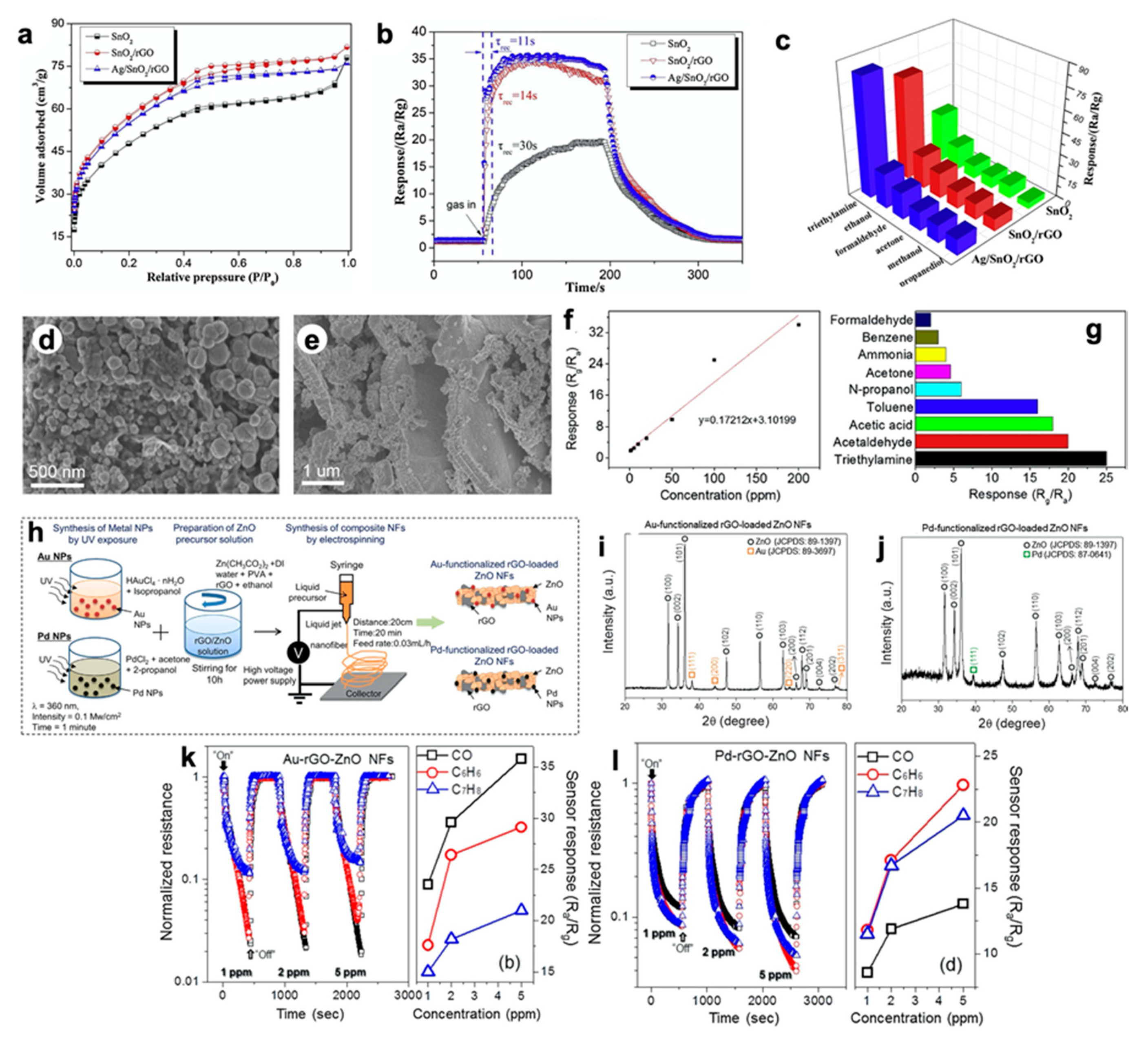
Divide the micromoles of gas pollutant by 44.615 to get micromoles per mole, or ppm. (Molar volume at "normal" conditions is 22.414 L). The normal cubic meter of air mixture is 44.615 moles. Divide the milligrams of gas pollutant by the molecular weight, and multiply by 1000 to express in micromoles. Convert the volume to other pressure and temperature by Ideal gas Law, and divide the new volume into the milligrams of particulate.įor gas pollutants, to express in ppm, both the pollutant and the air mixture must be converted to moles. At other temperatures and pressures, it will get larger or smaller, but still have the same milligrams of particulate inside. However, imagine a control volume surrounding the "normal" cubic meter. I'm Portuguese, and I don't write a lot in English.For the particulates, what conditions do you wish to reference them to? You have already measured at "normal" conditions. P.S.: Please forgive if I have written any word wrong. This questions I've made are related to the work that I have been doing, which is the control of pollutant emissions, in the plant that I work. I have another doubt, which is: for gas pollutants like NOx, CO, COV, etc, measured at the same conditions mencioned above, how could I convert to ppm?And for tons? The value obtained for particules was 33.1mg/Nm3. The condition of the sampling and analisys were, P=101.3 kPA and T=0✬.

How can we convert mg/Nm3 for particules. I'm reading your discussions in this forum, and I have a few questions.


 0 kommentar(er)
0 kommentar(er)
